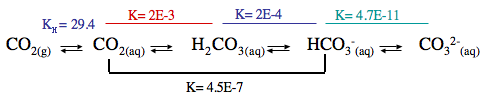Shrimphaus is usually around pH 8.2
The Shrimphaus has an equilibrium pH of around 8.2 which is on the alkaline side. Chemistry and geology of Cambridgeshire water makes this the case, discussed below.
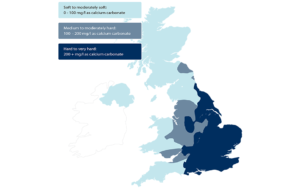
Chalk aquifers mean hard, alkaline water
Cambridgeshire water flows through chalk (calcium carbonate: CaCO3) aquifers making the water very hard. Carbon dioxide from the air dissolves in water converting to a small degree into carbonic acid, which subsequently reacts with and dissolves the chalk, giving both free calcium ions and bicarbonate.
CaCO3 (s) + CO2 (aq) + H2O (l) ⇌ Ca2+ (aq) + 2 HCO3− (aq)
Calcium (and magnesium) ions are components of general hardness (dGH) whilst bicarbonate ions make up the other type of hardness, carbonate hardness (dKH), also known as ‘alkalinity’. Cambridgeshire water is high in both types of hardness with dGH and dKH both around 17 units. It is the dKH, the alkalinity of the water, that is driving the high pH.
Atmospheric CO2, alkalinity and pH equilibrium in water
The bicarbonate system in water is a quite complex balance between atmospheric CO2, dissolved CO2, carbonic acid, bicarbonate and carbonate.
In the water, the balance between these different chemical species is determined by pH and the reverse is also true. This pH dependence on the relative abundance of the different chemical species in a closed system can be calculated and plotted.
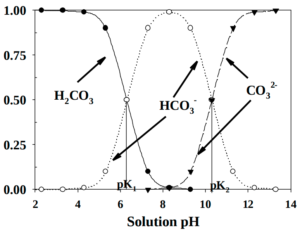
The actual distribution of species in a system open to the atmosphere adds another level of complexity where with alkaline pH water, dissolved inorganic carbon (DIC) increases exponentially.
Aquarium pH dependencies

The pH of the water turns out to be almost entirely dependent on:
- carbonate hardness (alkalinity, dKH) – more carbonate hardness causes increased pH (and vice versa)
- the amount of dissolved CO2 (composite carbonic acid) – more dissolved CO2 decreases pH
The carbonate hardness is set by the water used for water changes, for the Shrimphaus that is around 17 dKH. The amount of dissolved CO2 in the water is driven to an indoor equilibrium of between 1 and 3 ppm. This equilibrium results from the fixed partitioning ratio between atmospheric and dissolved CO2 – this fixed ratio is known as Henry’s law.
The day after a water change in the Shrimphaus the pH is 8.23, the alkalinity is 246 ppm CaCO3 and the conductivity is 644 µS/cm (TDS of 322). The pH immediately after the water change was 7.81 which is temporarily lower than the equilibrium value due to additional CO2 dissolved in the pressurised mains water supply which is lost to outgassing to atmosphere overnight.
Alkaline water impact on plants and animals
Water that is both alkaline (high dKH) and hard (high dGH) has a reputation for making submersed plant growth difficult. I will say that many types of submersed plants have done quite badly in the Shrimphaus. The double challenge of low CO2 (no CO2 gas injection) and hard water with high pH seems to have restricted the number of species that can thrive. So far Alternanthera reineckii ‘Mini’ and Helanthium bolivianum ‘Quadricostatus’ have done convincingly well, and some types of cryptocoryne species have also done pretty good. It is also definitely possible to grow black beard algae under alkaline water conditions – fortunately that has retreated recently as a problem in the Shrimphaus.
Shrimphaus doesn’t have any fish, just bloody mary shrimp. They seem unfazed by the water conditions and live, eat and breed productively. Copepods and snails likewise haven’t had any noticeable problems – actually snails can be harmed by acidic aquarium water.