

A drop checker is a simple and reliable device for measuring the level of dissolved CO2 in aquarium water. The drop checker hangs from the wall of the tank inside the tank and completely underwater, e.g. with a suction cup, and contains a liquid with a pH indicator dye in the “drop” portion which is connected to an open interface to the aquarium water at the bottom while maintaining an air gap in a U-bend between the aquarium water and the pH indicator solution. There is an equilibrium between CO2 in solution in the water (in the form of carbonic acid) and the gas phase CO2 in the air gap. The CO2 in the air gap can dissolve back into the aquarium water and equivalently into the drop checker solution such that eventually a steady state is reached where the concentration of carbonic acid in the aquarium and the concentration of carbonic acid in the pH indicator will be the same when then the amount of carbonic acid in the aquarium can be inferred directly from the colour of the pH indicator solution*. Usually bromothymol blue is the pH indicator and the drop checker solution is bromothymol blue diluted into a defined concentration of sodium bicarbonate at typically a “hardness” of 4 dKH. It is convenient to purchase pre-made bromothymol blue in a 4 dKH solution that can be added directly into the drop checker. We’re looking for as much dissolved CO2 as possible to support plant growth without have so much CO2 that it adversely affects animal life, generally accepted to be around 30 parts per million (PPM) dissolved CO2. Under a typical drop checker setup with 4 dKH solution, a blue colour indicates not enough CO2, green indicates the right amount of CO2, and yellow means too much CO2.
Measuring aquarium CO2 with a drop checker
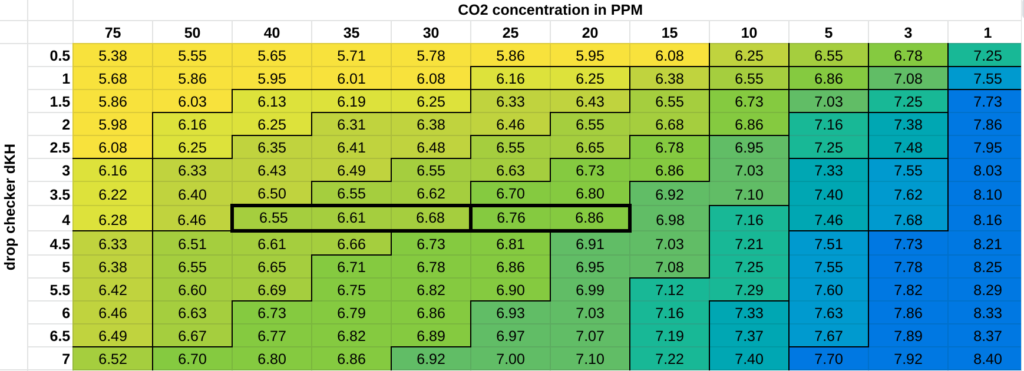
Here’s a chart I made that shows what colour the bromothymol blue solution in the drop checker will be, shown as a function of hardness of the water in the drop checker (usually set to 4 dKH – shown as the row headings) and the concentration of dissolved CO2 in the aquarium water (shown in the column headings). People generally aim for 30 parts per million (PPM) CO2 dissolved in the water, so if you read the chart across at 4 dKH you can see how the central lighter and darker green colours (outlined in bold boxes) indicate a CO2 concentration of beween 20 – 40 PPM, which is generally fine. The numbers inside the coloured boxes are the pH of the drop checker solution at equilibrium, which again is not the same as the pH of the aquarium water.
The older way to measure dissolved CO2 was to measure the pH of the aquarium water directly and to measure the dKH of the aquarium water directly, where you could then read off the dissolved CO2 levels from a pre-calculated chart. Since it is quite difficult to get an accurate reading of either aquarium water pH or dKH, the drop checker solves these difficulties by having a defined dKH in the drop checker solution and using a very sensitive pH indicator dye.
Tips for easy and accurate use of a drop checker

- Do not use aquarium water as drop checker solution! To be accurate, the drop checker solution needs to contain a defined level of dissolved bicarbonate in pure water with pH indicator dye and nothing else. It is worth spending a few pennies to purchase a small bottle of commercially-made drop checker solution.
- It generally takes a couple of hours at least for the drop checker to reach equilibrium CO2 levels with the aquarium water, so this is not a “rapid response” test – you leave the drop checker in the tank all the time.
- Reading the colour of the drop checker against a white background can be helpful. The left-side picture above uses a white credit card blank held up as a background – these credit card blanks also make great non-scratching algae scrapers for acrylic aquarium walls.
- Dennerle also makes a very nice drop checker with a built-in white reference backdrop.
- At low CO2 the blue colour of the drop checker solution is also opaque. As the drop checker becomes progressively more green towards yellow as CO2 levels rise, the solution will also become transparent so you’ll be able to see details in things behind the drop checker. The right side real-world drop checker picture above exhibits this type of transparency.
- At water change time be sure the bottom surface of the drop checker hasn’t become covered over with biofilm. For some reason a whitish residue can build up where the air gap in the drop checker meets the aquarium water. If this happens the free exchange of CO2 in and out of the air gap can be compromised, in which case give your drop checker a good clean out and replace with fresh drop checker solution.
- There are some instances of fish gulping the air out of the bottom of a drop checker, so if it seems like your drop checker is “leaking” this might be worth exploring.
Measuring drop checker colour with a colour-checking app
For example for hobbists with colour blindness, if you have an illuminated white background behind the tank and look through the in-tank drop checker at this illuminated white background, there are really great mobile phone apps that will reliably measure the colour of the drop checker. I use Color Grab and have found the hue reading (H in HSV) is a straightforward indicator of drop checker pH. For a standard 4 dKH drop checker solution in a 25C aquarium, you can use the table below to read off the pH of the drop checker and thereby the CO2 concentration of the aquarium water from the hue of the drop checker as shown by the app.
| Hue (H of decimal HSV) | pH of 4 dKH drop checker solution | CO2 in aquarium water (ppm) |
| 57 | 6.0 | 140 |
| 64 | 6.2 | 88 |
| 69 | 6.4 | 56 |
| 76 | 6.6 | 35 |
| 86 | 6.8 | 22 |
| 112 | 7.0 | 14 |
| 161 | 7.2 | 9 |
| 187 | 7.4 | 6 |
| 202 | 7.6 | 4 |
| 216 | 7.8 | 2 |
I aim for CO2 in the 35-40 ppm range which would be hue values around 74 or 75.
Sources
- https://www.aquaticplantcentral.com/threads/is-the-ph-kh-co2-equation-completely-wrong.2222/
- a revisitation of the calculations correcting an error very highly propagated across websites
- http://iyc2011.iupac.org/system/documents/107/original/pH_of_the_Planet_25-1-2011.pdf
- nice description of bromothymol pH indicator and how to use it
- has an RGB table describing bromothymol blue colour at various defined pH points
*Even though at equilibrium the concentration of carbonic acid in the drop checker is the same as the concentration of carbonic acid in the aquarium water, the pH of the drop checker solution is not the same as the pH of the aquarium water. This is because the pH of the aquarium water is influenced by many factors other than carbonic acid none of which affect the drop checker, and because the pH is also strongly influenced by the “hardness” of the water which is always 4 dKH in the drop checker but could be almost anything in the aquarium.